Synthetic Chemistry
We strive to bring the power of good chemistry to biology and materials science, so synthetic organic chemistry is the foundation of our research program. The chemists in our lab contribute to many projects by making covalent linkers for bioconjugation, small-molecule targets (haptens) and molecular adjuvants for immunology, protein-binding agents as biochemical probes, oligopeptides, monomers for polymer chemistry, and carbohydrates and oxanorbornadienes as described elsewhere on this page. We also develop bioconjugation and click chemistry methodologies, recently focused on new methods for reversible labeling of nitrogen nucleophiles in proteins and on specialized ligands for copper-catalyzed azide-alkyne cycloaddition.
Drug Delivery and Materials with Oxa/Aza Norbornadienes
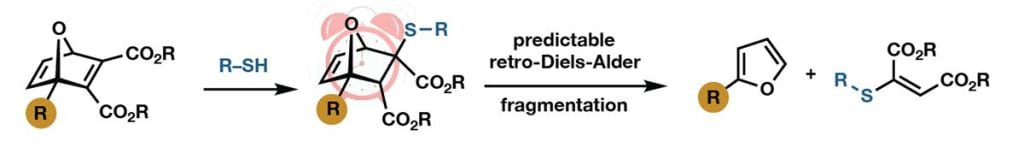
The 7-oxanorbornadienes (ONDs), derived from Diels-Alder cycloaddition of furans and electron-deficient alkynes, are an easily synthesized class of compounds that serve as highly reactive electrophiles and interesting cleavable linkages. Potent Michael acceptors, this class of molecules is roughly reactive as maleimides toward conjugate addition of thiols, yet more stable toward deactivation in aqueous solution. Most exciting is their ability to undergo retro-Diels-Alder fragmentation at rates that are controllable over a remarkably wide range, with half-lives from minutes to months. We continue to develop the fundamental chemistry of these systems, and apply them to the creation of degradable hydrogels, drug delivery agents, and other functional materials. Ongoing projects include: kinetic studies towards a fundamental understanding of cleavage rates, development of linkers derived from pyrroles rather than furans (azanorbornadienes), multi-functionalized systems for programmed non-burst release, OND–nanoparticle conjugates for targeted delivery to the lymph nodes for immune cell activation, and further development of biocompatible, drug-releasing hydrogels.
Carbohydrate Chemistry
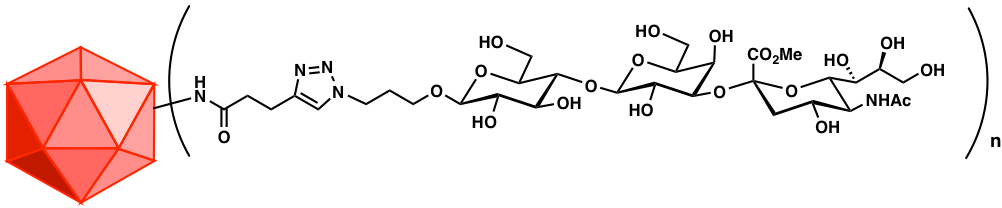
Carbohydrates constitute the most information-rich class of molecules in biology. All cells display large quantities of carbohydrates, often with unique composition for each species, life stage, or even cell state. They would make for outstanding targets for the immune system but for one problem: bearing mostly functional groups no more complex than hydroxyl and N-acetyl, although in limitless structural complexity, glycans are usually poorly immunogenic. We have developed virus-like particle-based methods to dramatically enhance the immune response against carbohydrates. To fully harness the power of this technique requires the chemical synthesis of these complex molecules in forms adapted for conjugation to various protein-based platforms. We therefore make disease-relevant glycans, mostly di-, tri-, and tetrasaccharides, focusing on unique epitopes from parasites, bacteria, and cancer cells. This work uses state-of-the-art multistep methods to prepare antigens and complementary analytical reagents. We also collaborate with carbohydrate chemists in several other institutions on these exciting targets.