Kyle Allison’s Lab combines microscopy, genetics, and quantitative analyses to investigate the dynamic behaviors of bacterial cells. The Allison Lab has made discoveries in the metabolite potentiation of antibiotics, the resuscitation of persistent bacteria, and the multicellularity and multicellular self-organization of E. coli (the best-studied unicellular organism).
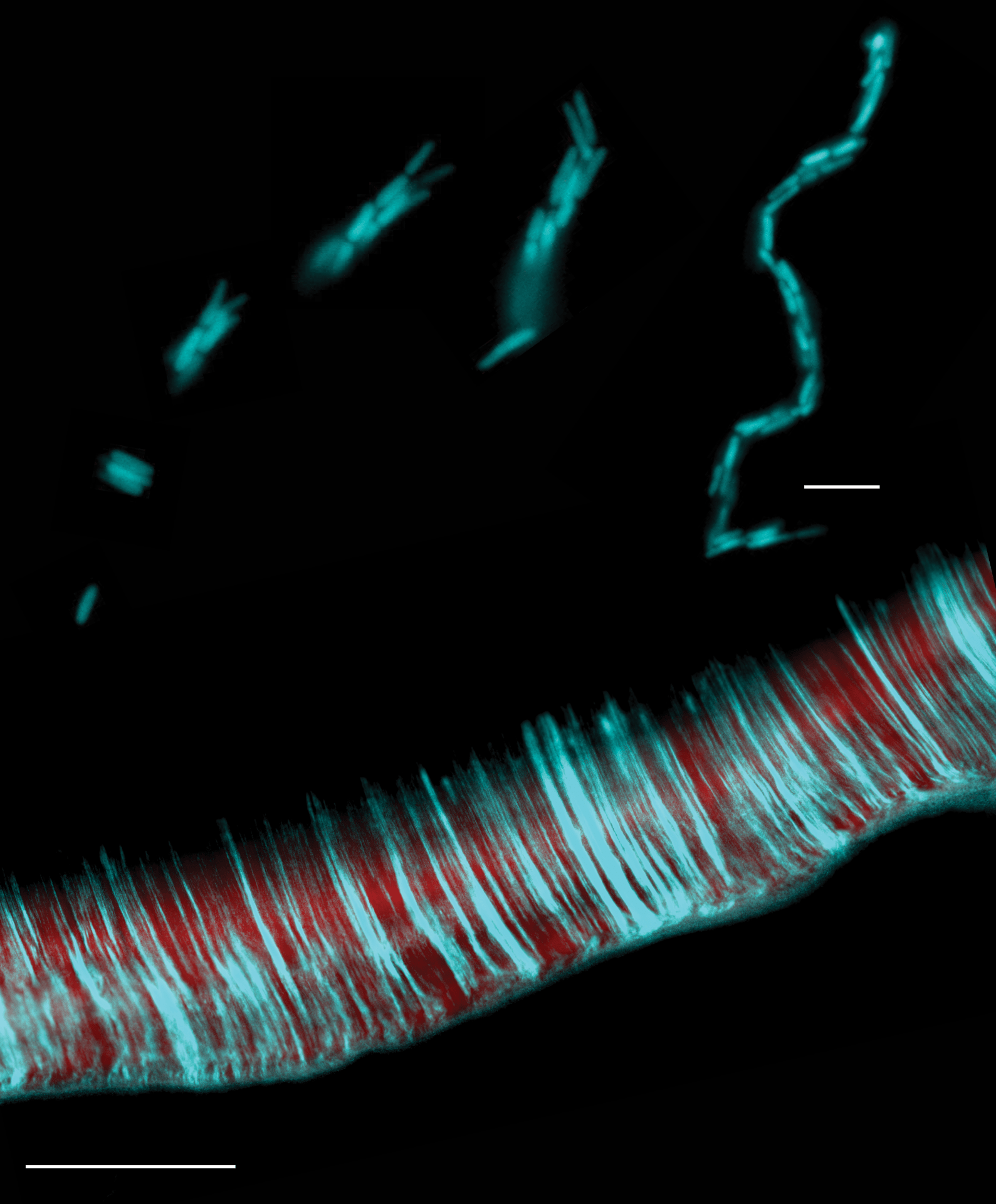
Bacterial biofilms are multicellular communities that contribute to infections. Though their genetics have been studied, cellular-scale observations of their development are generally lacking. The above image depicts microscope images of a novel process of multicellular self-organization in E. coli (above; scale bar 10 μm) and the resulting biofilm grown from a mixture of red and green (cyan) cells (below; scale bar is 500 μm). Puri et. al., tracked the entirety of morphogenesis in Escherichia coli at the cellular-scale and report a novel multicellular developmental process, initiated by the formation of 4-cell rosettes that then grow into ~1,000 chain-like communities before attaching to surfaces as clonal units. An accompanying new method demonstrated that the parallel-aligned accumulation generates biofilms. This process has multiple distinct stages each of which is genetically-regulated. This study establishes that E. coli, a unicellular bacterium, can follow a clonal self-organizing multicellular life cycle, that unfolds entirely at the microscopic scale.